Mikrogen Diagnostik recomWell Chlamydia pneumoniae IgG ELISA Kit (6104)
$462.00
SKU: 6104
Categories: Infectious Disease Test Kits and Positive Controls, MIKROGEN Products
Overview
Product Name Mikrogen Diagnostik recomWell Chlamydia pneumoniae IgG ELISA Kit (6104)
Description Enzyme immunoassay based on Chlamydia pneumoniae specific antigen (COMC) for the detection of IgG, IgM or IgA antibodies against Chlamydia pneumoniae in human serum and plasma
Product is intended for research use.
Product is intended for research use.
Target Human Chlamydia pneumoniae IgG
Species Reactivity Chlamydia pneumoniae
Assay Type Indirect ELISA
Applications ELISA
Associated Products recomWell Chlamydia pneumoniae IgM ELISA Kit (6105)
recomWell Chlamydia pneumoniae IgA ELISA Kit (6106)
recomLine Chlamydia IgG Lateral Strip Test Kit (6172)
recomLine Chlamydia IgA (IgM) Lateral Strip Test Kit (6173)
recomWell Chlamydia trachomatis IgG ELISA Kit (6904)
recomWell Chlamydia trachomatis IgA ELISA Kit (6905)
Chlamydia pneumoniae IgA Control (C1371A)
Chlamydia pneumoniae IgG Control (C1371G)
Chlamydia pneumoniae IgM Control (C1371M)
Chlamydia trachomatis IgA Control Serum (C1372A)
Chlamydia trachomatis IgG Control Serum (C1372G)
Chlamydia trachomatis IgM Control Serum (C1372M)
Chlamydia pneumoniae IgA ELISA Kit (ESR1371A)
Chlamydia pneumoniae IgG ELISA Kit (ESR1371G)
Chlamydia pneumoniae IgM ELISA Kit (ESR1371M)
Chlamydia trachomatis IgA ELISA Kit (ESR1372A)
Chlamydia trachomatis IgG ELISA Kit (ESR1372G)
Chlamydia trachomatis IgM ELISA Kit (ESR1372M)
Chlamydia IgA ELISA Kit (ESR137A)
Chlamydia IgG ELISA Kit (ESR137G)
recomWell Chlamydia pneumoniae IgA ELISA Kit (6106)
recomLine Chlamydia IgG Lateral Strip Test Kit (6172)
recomLine Chlamydia IgA (IgM) Lateral Strip Test Kit (6173)
recomWell Chlamydia trachomatis IgG ELISA Kit (6904)
recomWell Chlamydia trachomatis IgA ELISA Kit (6905)
Chlamydia pneumoniae IgA Control (C1371A)
Chlamydia pneumoniae IgG Control (C1371G)
Chlamydia pneumoniae IgM Control (C1371M)
Chlamydia trachomatis IgA Control Serum (C1372A)
Chlamydia trachomatis IgG Control Serum (C1372G)
Chlamydia trachomatis IgM Control Serum (C1372M)
Chlamydia pneumoniae IgA ELISA Kit (ESR1371A)
Chlamydia pneumoniae IgG ELISA Kit (ESR1371G)
Chlamydia pneumoniae IgM ELISA Kit (ESR1371M)
Chlamydia trachomatis IgA ELISA Kit (ESR1372A)
Chlamydia trachomatis IgG ELISA Kit (ESR1372G)
Chlamydia trachomatis IgM ELISA Kit (ESR1372M)
Chlamydia IgA ELISA Kit (ESR137A)
Chlamydia IgG ELISA Kit (ESR137G)
Properties
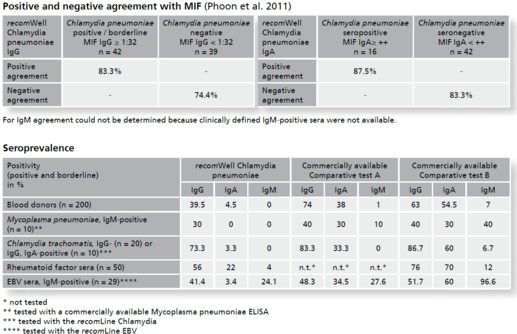
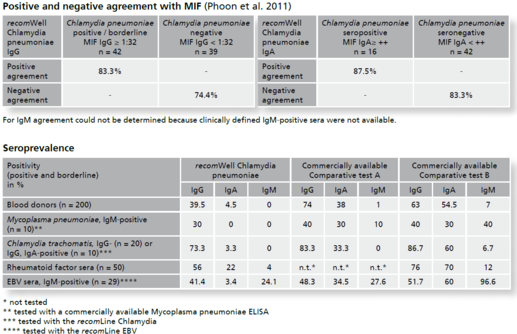
Background Chlamydia pneumoniae is a widespread, obligately intracellular bacterium which affects the respiratory tract. In Germany the prevalence in women and men in the sixth decade of life is estimated >50% and >70% respectively. Most infections are asymptomatic, a few infections cause mild symptoms like sinusitis or bronchitis. Nevertheless, we also see severe courses of infections associated with asthma, pneu-moniae, rarely coming along with arthritis or atherosclerosis. Chlamydiae reproduce in a biphasic development cycle showing extracellular, infectious elementary bodies and intracellular, metabolically active but not infectious reticular bodies. This kind of reproduction facilitates persistence formation and chronical infection.In diagnostics direct detection of chlamydia antigen (cultivation and PCR techniques) and indirect proof by antibody detection are used. Cultivation of Chlamydiae however is quite time-consuming and PCR is not well standardized. Reliable differentiation between acute and persistent infection remains difficult. In Chlamydia pneumoniae serology the Micro Immune Fluorescence (MIF) assay is considered as the gold standard. This technique is labor-intensive, requires experience and is therefore reserved for special laboratories. To avoid unnecessary antibiotic therapy there is a need to provide accurate analytics for routine diagnostics to be guided by the gold standard MIF and reflecting the clinical situation.To detect antibodies against the pathogen the recomWell Chlamydia pneumoniae is using antigens from the outer membrane complex of Chlamydia pneumoniae (COMC). With the objective to discriminate mild, clini-cally inconspicuous infections from clinically relevant infections the test was built up on a MIF cut-off value of ≥1:32. The recomWell Chlamydia pneumoniae shows an excellent specificity compared to other commercially available ELISA test systems. In healthy blood donors the recomWell Chlamydia pneumoniae detects an IgG prevalence of 39.5% and an IgA prevalence of 4.5% respectively.
Sample Type Serum, plasma, whole blood
Assay Resolution See Figure
Components
| 10X Wash Buffer | 100 mL |
| Dilution Buffer | 125 mL |
| TMB Substrate | 12 mL |
| Stop Slution | 12 mL |
| Instuctions for Use | 1 Each |
| Evaluation Form | 1 Each |
| Microplate Sealing Tape | 2 Each |
| Microtiter Plate | 12 x 8 Wells |
| Positive Control | 450 uL |
| Cut-Off Control | 450 uL |
| Negative Control | 450 uL |
| Anti-Human IgG Conjugate | 500 uL |
Specificity Information
Target ID Human Chlamydia pneumoniae IgG
Research Areas Infectious Disease
Application Images



Description Test principle and procedure.

Description Test evaluation data.
Additional Information
Additional Information Additional Mirkogen Kit Information
Handling
Storage Store at 2-8°C.
References & Data Sheet
Data Sheet  Download PDF Data Sheet
Download PDF Data Sheet
 Download PDF Data Sheet
Download PDF Data Sheet